Clinical Roots
A Decentralized Clinical Trial Management System
Transforming clinical trial recruitment with rapid data quality enhancement
Explore Clinical Roots, an advanced system crafted for the clinical research community, designed to enhance data quality swiftly and effectively.
Offering robust trial validation and precise data accuracy, this platform promotes seamless integration within an expansive, interconnected ecosystem, tailored specifically for the needs of health institutions.
Advanced clinical trial validation and management
Clinical Roots is leading the transformation of clinical trial management by providing an advanced system that ensures the accuracy and reliability of trial information. Our latest feature enables users to validate and verify clinical trial data, ensuring that it is current and certified by trusted organizations. This is crucial for research teams and pharma companies, helping them overcome recruitment challenges and ensuring the integrity of trial data.
Take charge of your clinical trials
With Clinical Roots, research organizations and pharma companies gain unprecedented control over their clinical trials. Our platform enables you to:
Ensure Data Integrity
Validate trial information with certifications, ensuring the data meets the highest standards of accuracy.
Monitor Key Metrics
Access critical KPIs to track trial progress, identify bottlenecks, and optimize outcomes.
Facilitate Recruitment
By ensuring that data is accurate and up-to-date, we help remove obstacles that could hinder participant recruitment.
Enhancing the experience for health professionals and patients
For health professionals, patients, and caregivers, Clinical Roots offers a powerful advantage. The ability to filter and prioritize recently updated and validated trials significantly improves the chances of participation. This transparency and accuracy empower stakeholders to make informed decisions about clinical trial involvement.
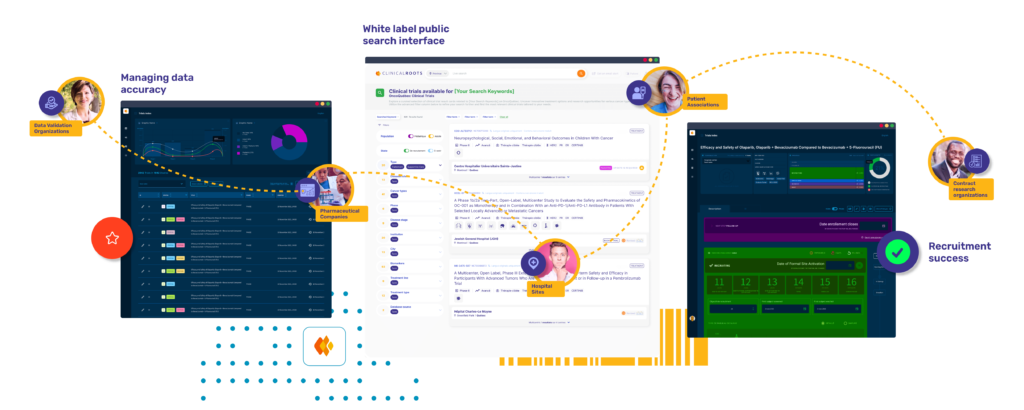
Benefits of the Clinical Roots White Label System
By using Clinical Roots as a white label solution, you maintain complete control over the user experience while leveraging the platform’s powerful suite of features to enhance clinical research operations.
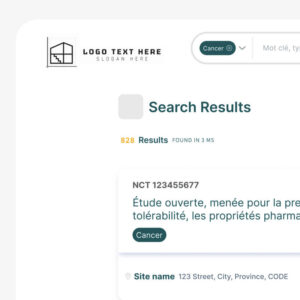
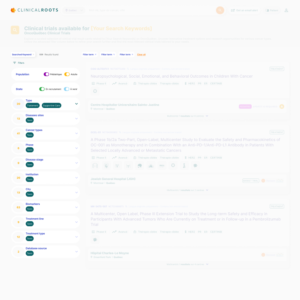
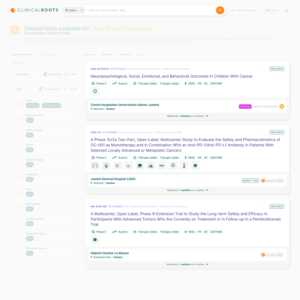
The Public Trial Listing Interface
The Public Trial Listing Interface serves as the outward-facing portion of Clinical Roots, where clinical trials are displayed to the public.
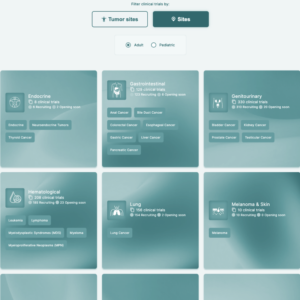
This customizable and user-friendly platform allows potential participants, health professionals, and caregivers to search for trials based on a variety of filters, such as regional hospital locations, disease types, populations, and biomarkers.
With multilingual support, the front office ensures that clinical trials are accessible to a diverse audience.
The real-time trial information and advanced filtering options make it easier for patients and professionals alike to find trials that meet their needs, improving the overall recruitment experience.
Live search
The Clinical Roots front office includes a live text search function that allows users to easily find clinical trials by adding any type of term association. This is useful for both health professionals looking for specific trials and for patients searching for trials related to their specific condition.
A customizable White Label trial management solution for your organization
Clinical Roots offers a flexible White Label Solution, allowing organizations to implement the platform with their own branding, colors, and identity. While the interface is tailored to your organization’s unique look and feel, the robust core features of Clinical Roots remain intact, providing powerful tools for trial validation, management, and data accuracy. This white label capability ensures that organizations can promote their own distinct presence while benefiting from a proven and efficient clinical trial infrastructure.

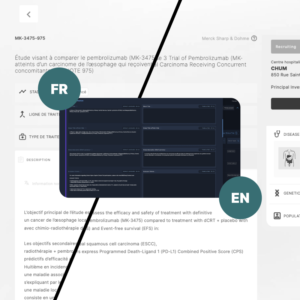
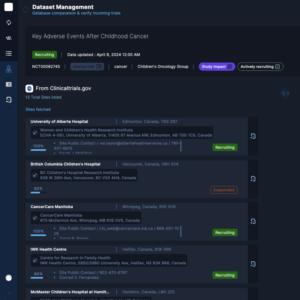
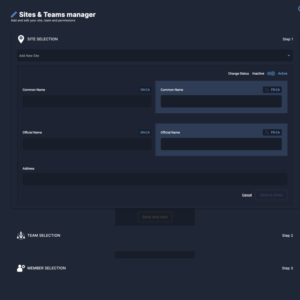
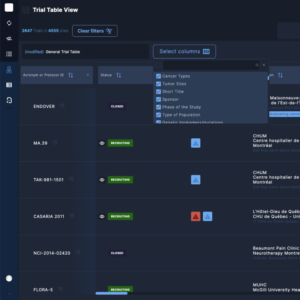
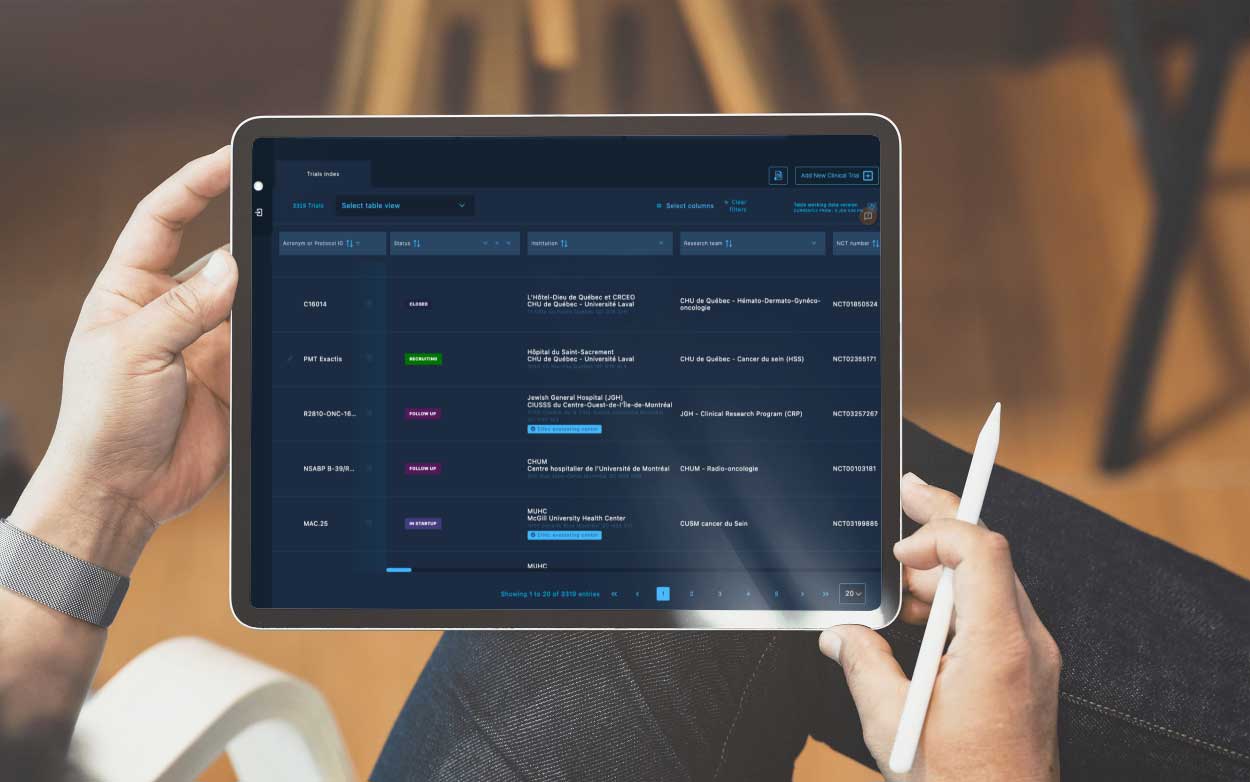
The Private administrative interface
The Private Administrative Interface is the behind-the-scenes engine driving Clinical Roots’ trial management capabilities.
This secure and robust platform empowers research teams and administrators with tools to manage and update trial information, monitor key performance indicators (KPIs), assign roles to team members, and oversee multiple sites from one centralized location.
The back office enables greater accuracy in data tracking and provides full control over the trial validation process. It also supports the integration of data from clinicaltrials.gov, automating the flow of critical information.
By maintaining a centralized control panel, the back office enhances collaboration, efficiency, and data-driven decision-making throughout the clinical trial lifecycle.
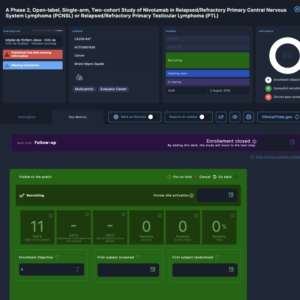
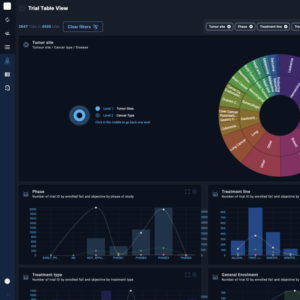
Powerful KPIs
The Clinical Roots back office includes powerful key performance indicators (KPIs), providing a quick performance overview for health professionals managing clinical trials. These KPIs allow users to track and analyze important metrics such as enrollment numbers, completion rates, and patient engagement. By presenting this data in an easy-to-understand format, the Clinical Roots back office helps health professionals stay informed and make data-driven decisions about their clinical trials. The back office also includes customizable options for the KPIs, allowing users to focus on the specific metrics that are most important to their specific trial.
A cohesive ecosystem for seamless collaboration
Clinical Roots is designed to operate within a fully integrated and interconnected ecosystem, facilitating collaboration between diverse teams and institutions involved in the clinical research process. From Contract Research Organizations (CROs) and pharmaceutical companies to hospitals, patient associations, and research support organizations, Clinical Roots connects all stakeholders in a shared environment. This ensures seamless data management, transparent communication, and operational efficiency across the entire clinical trial lifecycle, empowering all parties to contribute effectively to advancing healthcare innovation.
Key benefits
Team Management
Collaborative Workload Management
Assign dedicated teams to oversee and verify multiple clinical trials, fostering effective collaboration and streamlined operations.
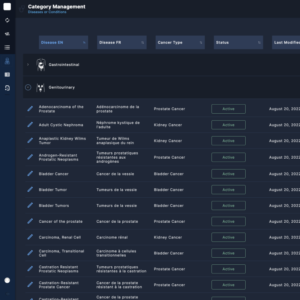
Site Management
Accurate and Up-to-Date Information
Regional centers can easily manage and update the contact information for hospital centers, ensuring accurate communication and coordination within their region.
User Management
Efficient Role Management
Manage user contact information and roles effortlessly, ensuring that each team member's responsibilities are clearly defined and aligned with the trial objectives.
Multilingual Support
Bilingual Accessibility
Our platform supports both English and French, catering to the diverse linguistic needs of Canadian stakeholders and ensuring that Clinical Roots is accessible to all users.
Embeddable Widget
Customizable and Responsive Integration
Clinical Roots offers an embeddable widget that is easy to install on any HTML page. Each member of your organization can generate a personalized version of the widget, ensuring that it integrates seamlessly with existing websites. The widget is fully responsive, providing an optimal user experience across all devices, and enhances trial visibility by making information accessible and searchable.
Case studies
- Oncology
- Quebec, Canada
- 18 Hospital centers
Clinical Roots is a proven and reliable system that has been successfully used in the province of Quebec since 2019, supporting more than 20 hospitals involved in oncology clinical trials.
The system was designed with direct input from health professionals, clinical trial researchers, and trial managers, ensuring it addresses the real-world needs and challenges faced in oncology research. This collaborative approach has been instrumental in creating a platform that aligns with the complex demands of clinical trial management. Additionally, the Clinical Roots team conducted an ethical review of the development process to ensure the platform effectively addresses critical issues while delivering tangible results.
Leveraging its customizable white-label feature, Clinical Roots has been adapted to support oncology trial management across multiple institutions. Its flexible infrastructure enables seamless trial coordination, data validation, and participant recruitment, contributing to improved operational efficiency and enhanced interconnectivity within Quebec’s oncology research ecosystem.
Clinical Roots continues to demonstrate its value as an essential tool for advancing the management of oncology clinical trials, driving better outcomes through optimized processes and collaborative engagement.
Using Clinical Roots widget to promote a network
OncoQuebec Leverages Clinical Roots Widget to Improve Clinical Trial Visibility and Recruitment
OncoQuebec has seen significant benefits from using the Clinical Roots widget to promote their clinical trials. By embedding the widget, featuring a live text search bar, on various hospital websites, OncoQuebec has been able to increase the visibility and recruitment for their trials.
Users can easily search for relevant clinical trials using the widget, improving the chances of finding qualified participants. The Clinical Roots widget has a user-friendly interface and is fully customizable to fit the branding guidelines of individual organizations.
By using the Clinical Roots widget, OncoQuebec has been able to streamline their clinical trial promotion process and improve the efficiency and interconnectivity of the process.
Find a clinical trial
OncoQuébec
Embeddable widget
Our widget is a powerful and easy-to-use tool for promoting your clinical trials to a wider audience. Simply embed the widget, featuring a live text search bar, on any external website and start increasing the visibility of your trials. The Clinical Roots widget has a user-friendly interface and is fully customizable to fit your branding guidelines.